electron configuration for iron|electron configuration cheat sheet : Baguio Learn how to write the electron configuration of iron using the noble gas shortcut and the energy levels of the orbitals. See the answer, the long version, and the explanation with diagrams. Eric Knowles, the beloved ceramics expert and familiar face on Bargain Hunt and Antiques Roadshow, has been gracing our screens since the 1980s.His rise to fame began on Antiques Roadshow, leading .
electron configuration for iron,The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .
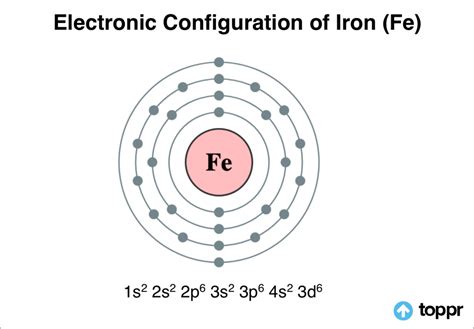
Electronic Configuration of Iron. The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a . In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons). When we write the configuration, we'll put all.
Learn how to write the electron configuration of iron using the noble gas shortcut and the energy levels of the orbitals. See the answer, the long version, and the explanation with diagrams.
The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, .Learn how to write the electronic configuration of iron and its oxidation states, valency, and applications. See examples, diagrams, and FAQs on iron's atomic structure and properties.
The electronic configuration of iron is represented as. Ar. Ar \[3d^{6} 4s^{2}\]. The typical crystalline framework and electronic configuration of iron make it usually .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .
Using the Aufbau Principle, the Pauli Exclusion Principle, and Hund's rule to predict an atom's electron configuration using the periodic table as a guide ; Differentiate between (spdf) electron configuration, orbital .
For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdf notation) is written as 1s 1 and read as “one-s-one. . The element is iron, Fe. b) 1s 2 2s 2 . We build electron configurations by filling the lowest energy orbitals first then filling progressively higher energy orbitals. This is known as the aufbau principal. . but imagine if you were .
Reduced Electronic Configuration of Iron is – [Ar] 3d6 4s2. The initial two electrons in the electron configuration for Iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, the next two electrons for Iron are in the 2s orbital. The following six electrons will enter the 2p orbital. Iron is a good example. Humans have known about iron long before we knew about elements and atoms. . The electron configuration is 4s 1, 3d 10 but all these general .
Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Iron (Fe) [Ar] 3d 6 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2: 2, 8, 14, 2: 27: Electron configuration of Cobalt (Co) [Ar] 3d 7 4s 2: 1s 2 2s 2 2p 6 3s 2 3p .Orbital diagram. Iron electron configuration. ← Electronic configurations of elements. Fe (Iron) is an element with position number 26 in the periodic table. Located in the IV period. Melting point: 1535 ℃. Density: 7.87 g/cm 3 . Electronic configuration of the Iron atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 .

To write the orbital diagram for the Iron (Fe) first we need to write the electron configuration for just Fe. To do that we need to find the number of elect.
electron configuration cheat sheet To write the orbital diagram for the Iron (Fe) first we need to write the electron configuration for just Fe. To do that we need to find the number of elect.
The iron’s electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Valence electrons are a single outer shell electron in an atom’s outer shell that is responsible for the atom’s chemical characteristics. In other words, a valence electron is an atom’s electron that may be transferred to or shared with another atom and is placed .electron configuration for iron The ground state electron configuration of Fe is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^6"4s"^2" For all but about 20 transition metals, the Aufbau diagram is a useful tool that helps to .For example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence .

Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.The chemical symbol for Iron is Fe. Electron Configuration and Oxidation States of Iron. Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Electron Configuration. The periodic .
The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. It looks something like this.Its 26 electrons are arranged in the configuration [Ar]3d 6 4s 2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of electrons can be ionized. Iron forms compounds mainly in the oxidation states +2 .
electron configuration for iron electron configuration cheat sheetIron has 26 electrons so its normal electron configuration would be: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. When we make a 3+ ion for Iron, we need to take the electrons from the outermost shell first so that would be the 4s shell NOT the 3d shell: Fe 3+ 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. One other note on writing electron configurations: A short cut. The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. Unlike other elements, iron exists at the oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment in spite of being reactive to water and oxygen.
electron configuration for iron|electron configuration cheat sheet
PH0 · unabbreviated electron configuration for iron
PH1 · shorthand electron configuration for iron
PH2 · ground state electron configuration for iron
PH3 · fe2+ electron configuration condensed
PH4 · fe +2 def how many electrons added
PH5 · electron configuration cheat sheet
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa